We provide expertise from different fields of engineering on projects in the pharmaceutical industry.
This best practice became a bible in the pharmaceutical industry for new computerised systems and equipment implementations. Its purpose is to guide design and verification in a way that documented evidence is provided for their fit-for-use status. Unfortunately, this also results in the misleading idea that with the creation of more paperwork better quality is achieved. Such misconceptions have a negative effect on implementation timelines, prices and are a risk to quality. That is why this best practice, when used properly, is a project management tool that ensures optimal end results. In such a way mistakes can be detected and corrected early in the building process
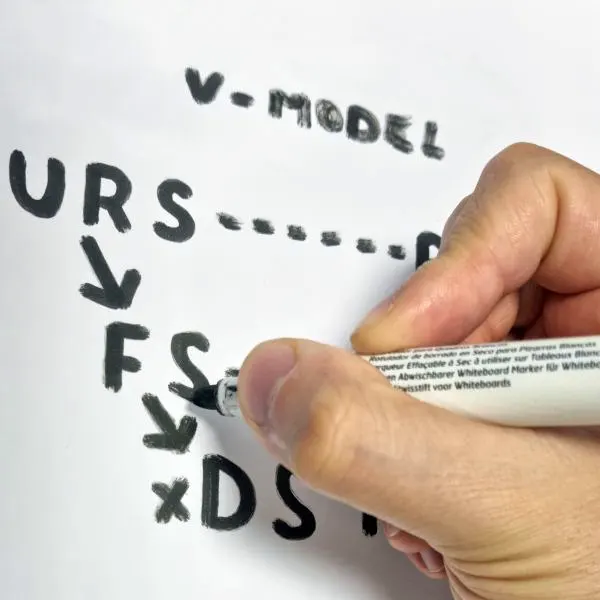
As in all aspects of the pharmaceutical industry regulations have a significant impact also on data used in the process of making pharmaceutical products. Whenever this data is generated, processed or stored in electronic form computerised systems are required to meet strict safety and security criteria. The main driver for this is FDA regulation CFR 21 part 11 intended to guide electronic records and electronic signatures measures when required by predicate rules described in CFR 21 parts 210 and 211. This complicated regulatory guidance often led to complex and costly systems. To overcome these challenges the right approach should be used to create an appropriate solution for each situation.

Counterfeiting of pharmaceutical products is big business and is growing each year. This threatens the health of millions around the world as people are not treated for their diseases or even poisoned with falsified drugs. Many countries introduced new anti-counterfeiting regulations to fight this. The most common concept is the use of serialisation as a unique identification measure for packed products. When combined with aggregation each packed product can also be easily located within any shipment. Ease of product verification by comparison of printed data with stored data in regulatory databases makes this a powerful tool that can prevent falsified medicines’ introduction to the market. As in many other situations, the pharmaceutical industry found a complex interdisciplinary solution. To implement a good working concept extended knowledge of processes, products, equipment, and IT systems is required.

Automation in industry uses machines and installations to connect them in a system intended to efficiently create new products. There are many ways to organise production as some products require continuous processes and others have batch processes, some even require job-oriented processes as each product can be unique. Whatever the process is, automated systems must be designed to ensure quality and efficiency. When we talk about how to achieve that, we usually start with the definition of process and data flows to find the optimal solution for the chosen production model. Best engineering practice teaches us to use modular and standardised concepts allowing easy exchange of segments to achieve efficient maintenance and prolonged lifetime. With the use of ISA/ANSI standards like S95 for interfaces between enterprise and control levels and S88 for batch operation models we have an excellent tool to achieve that.

Factories of the future will integrate new IT concepts like internet of things, big data, clouds, machine learning, blockchain, artificial intelligence, etc. This new industrial revolution, or Industry 4.0 as it is called, must be based on a good understanding of processes and interconnection of systems to achieve the high efficiency required of modern businesses. As with all new technologies introduced, costs can be enormous and that is why a smart approach to digitalisation requires good planning based on detailed process analyses and knowledge of best practice approaches to ensure its efficient implementation.

At CTRL-ING we provide efficient and quality-oriented pharmaceutical engineering consulting services.